Last week, my sister went to a birthday party
of her friend. She always put perfume when she is going to somewhere. So out of
curiosity, I checked the chemicals components inside a perfume and how it was
made. And this is what I gained so far. But the precise formulae of the
commercial perfumes are kept secret. The definition of perfume is a fragrant
liquid typically made from essential oils extracted from flowers and spices,
used to impart a pleasant smell to one's body or clothes. Perfume contains
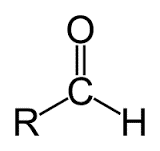
aldehydes group, the functional group consists of a carbon atom bonded to a
hydrogen atom with a single covalent bond and an oxygen atom with a double
bond. The general formula of an aldehyde is R-CH=O.
Aldehydes is vary in smell,
for example, formaldehyde and acetaldehyde have sharp and unpleasant odours.
But the common aldehydes used in perfumes is benzaldehyde and furfural, which
have pleasant and flowery odours, they are usually found in the essential oils
of certain plants. Some aldehydes have a very pleasant odours, and they can be
detected in a very low level of concentration. Floral aldehydes gives the
impression of the flowers like lavender, rose and jasmine. Because of these
characteristics, the aldehydes are used in the production of many perfumes. Production
of perfumes contain four main steps, which are Collecting, Extraction,
Blending, and Aging. Esters can be also found in perfume. They are the products
of the esterification reaction occuring between acids and alcohol. Water is
produced as a second product. When the molecular weight of small, they carry a
higher odour. They are resposible for the pleasant smell of apples, bananas,
strawberries. Saturated esters have a weaker odour than the unsaturated ones.
Making
of esters
Alcohol + organic acid → ester + water
They are 5 main property of perfume. First is
they are non-toxic, so that they does not poison the wearer. Secondly, the must
not irritate the skin, this is to prevent the wearer from suffering from
rashes. Thirdly, they are very colatile, they evaporates easily. This is
because the perfume can reach the nose easily. Next, they are insoluble in
water, so that it is not washed away easily. Lastly, they does not react with
water. This is to avoid the perfume reacting with sweat.
Authors : Aaron Leong Hee Lee, Huong Nai Zheng, Ong Kai Yin, Lim Huang Xu